A decade of dedication: our unwavering commitment to you and your patients
Explore our educational resources to learn more about ALPROLIX®
The Factor Forum is an engaging podcast series for healthcare professionals that provides peer insights and experiences that have helped them enhance treatment for their patients with hemophilia B. The series dives deep into topics, including treatment approaches, comorbidity management, patient cases, and the benefits of a multidisciplinary care team.
FACTORING IN SHLs AND EHLs FOR HEMOPHILIA B PATIENTS
Looks at the evolution of factor treatment and how EHLs have changed the hemophilia treatment landscape by optimizing care
AN APPROACH TO COMPLEX CASES IN HEMOPHILIA B
Explores common comorbidities and how choosing the right treatment regimen can impact patient outcomes and adherence
ALPROLIX in clinical and real-world settings
ALPROLIX Clinical Experience:
A conversation with Dr Amy Shapiro, MD, and Dr Lynn Malec, MD
Protection,* Experience,
and
Dosing:
A Discussion With Dr De Angulo
Access and reimbursement
Billing and
coding information
The codes listed below are provided for informational purposes only and are not intended to substitute for or influence the independent medical judgment of the prescriber. The codes may not apply to all patients or to all health plans, and additional codes not listed may apply to some patients. The treating physician is solely responsible for diagnosis, coding and determination of the appropriate ICD-10-CM codes that describe the patient’s condition and are supported by the medical record.
The ICD-10-CM and HCPCS codes provided are based on AMA or CMS guidelines. Use of the information below does not guarantee that the payor will provide coverage for ALPROLIX. Because government and other third-party payor coding requirements change periodically, please verify current coding requirements directly with the payor being billed.



This document contains information on how to use ALPROLIX, including reconstitution, pooling, administration, and storage.
ALPROLIX offers a range of financial assistance programs† for your patients
Access to these and other services is voluntary, and your patients are not obligated to begin treatment if they contact us. You and your patients make all treatment-related decisions.
Free Trial Plus Program
Patients can receive their first 30-day supply of ALPROLIX generally within 24-48 hours with a valid prescription from their healthcare provider. Sanofi Patient Services will review your patient’s health insurance information for their coverage of ALPROLIX while you and your patient decide if this is the right medication for them to continue taking.
Copay Program
Whether your patient is new to ALPROLIX or already on therapy, ALPROLIX offers a Copay Program to eligible patients that can cover up to $20,000 of copayment, or co-insurance, and out-of-pocket costs associated with their prescription. Best of all, there are no income requirements or caps, so your patient can get started with their treatment right away.
Learn more about Copay Savings for ALPROLIX .
Factor Access Program
Helps your patient access ALPROLIX, even if their insurance coverage is interrupted—for example, they are between jobs or changing insurers.
Sanofi Support Programs
Enrollment Form
Sanofi Support Programs Enrollment Form in
Spanish (en Español)
Sanofi Support Programs Bilingual Enrollment Form
†Copay Program not valid for patients utilizing Medicare, Medicaid, VA, DoD, TRICARE®, or similar federal or state programs including any state pharmaceutical assistance programs to pay in part or in full for their prescriptions. Savings may vary depending on patients’ out of pocket costs. Free Trial Plus valid only for a patient’s first prescriptions and it is limited to one use per patient per product for their lifetime. Free products dispensed through the Free Trial Plus or Factor Access Programs shall not be submitted to any third-party payer, public or private (e.g. private insurance, Medicaid, Medicare, VA, DoD, TRICARE, or similar federal or state programs) for reimbursement. All Programs not valid where prohibited by law. Sanofi reserves the right to modify or terminate the Programs at any time without notice. Program details provided upon registration.
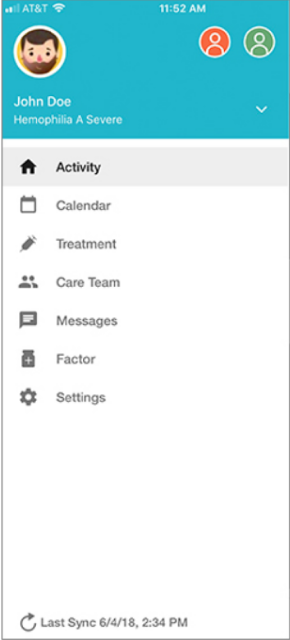
Patients can manage their hemophilia with the help of MicroHealth tracking
Sanofi has joined with MicroHealth in supporting patients and healthcare providers to better manage hemophilia.
Hemophilia B Advocacy Groups
This listing is provided as a resource only and does not constitute an endorsement by Sanofi of any particular organization or its programming. Additional resources on this topic may be available and should be investigated. Sanofi does not review or control the content of non-Sanofi websites. These listings do not constitute an endorsement by Sanofi of information provided by any other organizations.
The Coalition for Hemophilia B
National Hemophilia Foundation
 WATCH NOW:
WATCH NOW: